All Rare Earth Elements are not equal
Rare earth elements (REE) have occupied a quiet corner of the mining
industry since the late 1950s, but their unique properties have made
them crucial to a number of emerging and growing technologies,
increasing their demand and strategic importance.
The term “rare” is actually a misnomer and a carryover from
metallurgists from before 1950.
The metallurgical processes needed to isolate the individual
metal species are complex, and early technology prevented
commodity-level production. As a result, lanthanide metals or metal
oxides (i.e., REOs) were difficult to obtain and considered rare.
Actually some of them are relatively abundant in the earth’s
crust, but seldom occur in economic deposits.
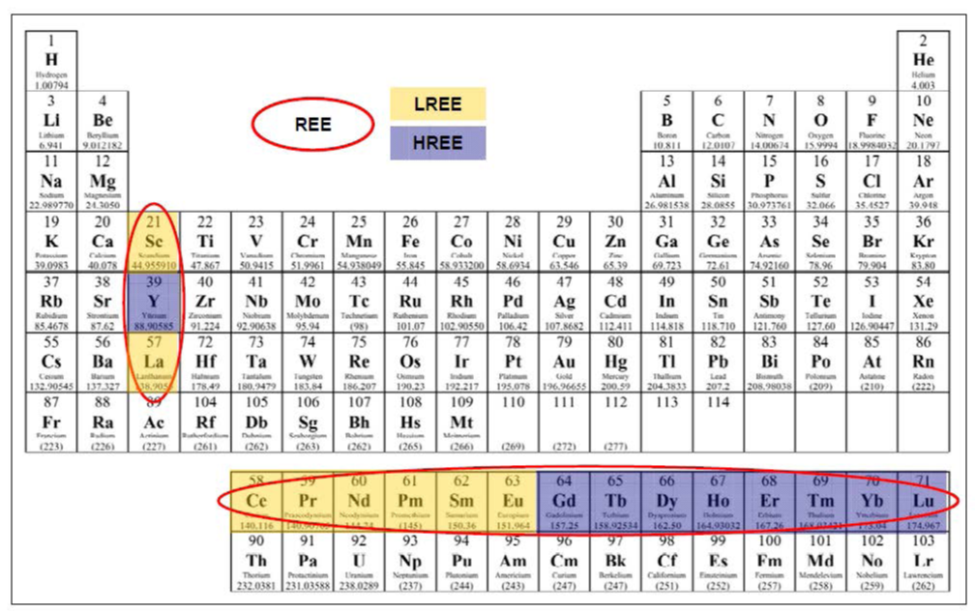
Officially, the International
Union of Pure and Applied Chemistry defines Rare Earth Elements as
the 15 Lanthanide elements, plus Scandium and Yttrium.
These 15 elements share common physiochemical properties:
Lanthanum (57La), Cerium (58Ce), Praseodymium (59Pr),
Neodymium (60Nd), Promethium (61Pm), Samarium (62Sm), Europium
(63Eu), Gadolinium (64Gd), Terbium (65Tb), Dysprosium (66Dy),
Holmium (67Ho), Erbium (68Er), Thulium (69Tm), Lutetium (71Lu),
Ytterbium (70Yb)
Due to their similar physiochemistry, these lanthanides often
occur together as elemental constituents of their host minerals. Two
other metals commonly found in association with lanthanides in the
same minerals and sharing similar physiochemical properties are:
Scandium (21Sc), Yttrium (39Y).
Following a common pattern within the periodic table, the
lanthanides with even atomic numbers are more common in nature.
Another observed pattern is in the relative abundance, REE
with lower atomic numbers are more common ionic constituents in REE
mineral ores and, in general, occurred in greater abundance than the
REE with higher atomic numbers.
This has led to a divide between light and heavy REE.
REEs do not occur as native elemental metals in nature, only
as part of the host mineral’s chemistry.
A rare earth mineral (REM) is a mineral which contains one or more rare earth elements as
major metal constituents.
Despite more than 200 known REE-bearing minerals, only three
are considered to be the principal REE mineral ores most feasible
for the extraction of REMs: bastnasite, xenotime, and monazite.

Bastnasite, the most abundant among the three REE mineral
ores, is a carbonate mineral found mainly enriched in LREEs (e.g.,
cerium, lanthanum, and yttrium). Bastnasite is found in vein
deposits, contact metamorphic zones, and pegmatites. It forms in
carbonate-silicate rocks occurring with and related to alkaline
intrusions (e.g., Mountain Pass mine).
The two phosphate minerals, xenotime and monazite, can occur
together, but crystallize in different temperature and pressure
regimes from a similar igneous environment. While these
minerals can contain any of the REEs (i.e., HREEs or LREEs),
enrichment of specific REEs is variable and a function of the
temperature and pressure regime in which they formed. Monazite
commonly occurs in placer deposits; xenotime can occur along with
monazite, but generally occurs as a more minor constituent of these
types of deposits. Deposits of phosphate rare earth ores provide the
opportunity to produce co-products of phosphates and REEs. Thorium
and uranium may also be taken advantage of and produced as a
co-product, or may represent a significant management challenge.

Monazite is generally enriched with the LREEs cerium,
lanthanum, and neodymium, but can also contain HREEs, particularly
yttrium. The predominance of LREEs is due to the lower
crystallization temperature and pressures of this mineral; however,
it typically contains more HREEs than bastnasite ore deposits. It
occurs in acidic igneous rocks (primarily pegmatites), metamorphic
rocks, and some vein deposits. Monazite is resistant to weathering
and occurs in many placer deposits as the host rocks are eroded.
Thorium may also be associated with monazite in various amounts.

Xenotime crystallizes under higher temperatures and pressures
than those of monazite; therefore, its crystalline structure more
readily accommodates a higher ratio of HREEs (terbium through
lutetium, and yttrium) than is commonly found in monazite. It is
primarily a yttrium phosphate mineral and occurs as a minor
constituent of granitic and gneissic rocks. Although not always
present in significant quantities, uranium and thorium can also
occur as constituents of xenotime.
There are two other important REE-containing minerals in the
United States; euxenite and allanite.

Euxenite which contains yttrium, erbium, and cerium. It is
found mostly in placer deposits in Idaho, and occurs as a
tantaloniobates (e.g., minerals where Ta and Nb form the compound)
of titanium, rare earths, thorium, and uranium.

Allanite is an epidote mineral and contains cerium,
lanthanum, and yttrium. It occurs in igneous, metamorphic, and
hydrothermal environments and is disseminated in pegmatite or occurs
in vein deposits.
These five minerals are considered to represent the principal
occurrences and the potentially more significant REE reserves in the
United States. However, many other minerals containing REEs do
occur, and deposits of these minerals could be found in the United
States and prove to be viable for mining. It is also not uncommon
for REEs to be produced as a coproduct or byproduct of other mineral
production.
The principal future domestic supply of REEs is one
carbonatite formation in Mountain Pass, California. Other common and
potentially viable deposit types containing almost exclusively the
two phosphate REE-bearing minerals (monazite and xenotime) are most
common as placer ores that originated from the erosion of pegmatite
granites and related gneisses.
Once the rare earth ore has been removed from the mineral deposit, the
difficult task of separating the different minerals into oxides
begins. Because the elements share similar characteristics,
the separation process is complex and it can vary from one mineral
resource to another. A deposit could contain a number of different
metals; but, with no processes available to extract them all a
company might have to focus on one element at the expense of
another.
Thorium, Uranium and other radioactive elements can also
contaminate Rare Earth Element deposits, making the elements more
expensive to recover. Once the different minerals have been refined
into oxides they are formed into rare earth alloys before they are
manufactured into their applications.








